Example: Kinetics - LHHW
The following example was taken from Ind. Eng. Chem. Res. 1992, 31, No. 1, pp. 107-119, called "Kinetics and Reaction Network in Propane Ammoxidation to Acrylonitrile on V - Sb - Al Based Mixed Oxides" Authors: Roberto Catani, Gabriele Centi, Ferruccio Trifiro and Robert K. Grasselli.
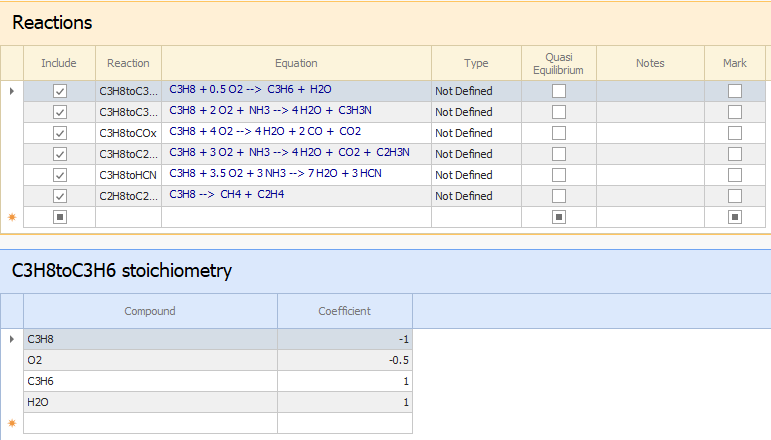
The first image displays the main reactions of the Propane Ammoxidation to Acrylonitrile, which were entered in the Reactions Node.
The next explanation will be for the first reaction but the procedure is similar for the rest of the reactions.
The kinetics information of the first reaction is as follows:
where
Forward direction only is enabled for all reaction in Chemistry → Kinetics node:
Then we define the kinetics parameters values for the reaction.
We first load the PreExponential and Activation Energy values for the parameter k that belongs to the numerator of the previous equation.
That is done in the Kinetics → Parameters node, as shown in the image below:
Notice that the propane, oxygen and ammonia orders are 1.
The following step is to define the sites, that is done in the LHHW Sites tab of Chemistry → Kinetics → Parameters node.
In this example, two sites are needed:
The first Site is: 1 + KC3H8CC3H8 , where:
The second site has two terms: Site2 = 1 + KO2CO2 + KNH3CNH3
Enter the first term :
Enter the second term:
After the Site definition is done, they must be associated with the appropriate reaction in Chemistry → Kinetics → Kinetics Sites node.
First, the sites are selected for reaction C3H8toC3H6 in the Kinetics Site tab:
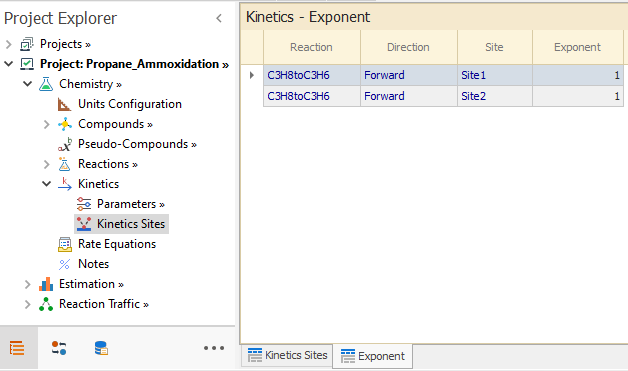
Finally, the exponent for each Site in the C3H8toC3H6 reaction is entered in the Exponents Tab:
This completes the kinetics setup for the first reaction.
The first image displays the main reactions of the Propane Ammoxidation to Acrylonitrile, which were entered in the Reactions Node.
|
|---|
The next explanation will be for the first reaction but the procedure is similar for the rest of the reactions.
The kinetics information of the first reaction is as follows:
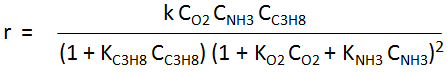
|
|---|
-
k is the kinetic constant
CO2, CNH3, CC3H8 are the concentration of oxygen, ammonia and propane respectively.
KO2, KNH3 and KC3H8 are the adsorption constants for oxygen, ammonia and propane respectively.
- k = 1.06045E+12 exp (-23.196/RT)
- KO2 = 6.21314E-1 exp (29.0377/RT)
- KNH3 = 3.81963E-1 exp (32.4/RT)
- KC3H8 = 1.80516E-1 exp (24.4269/RT)
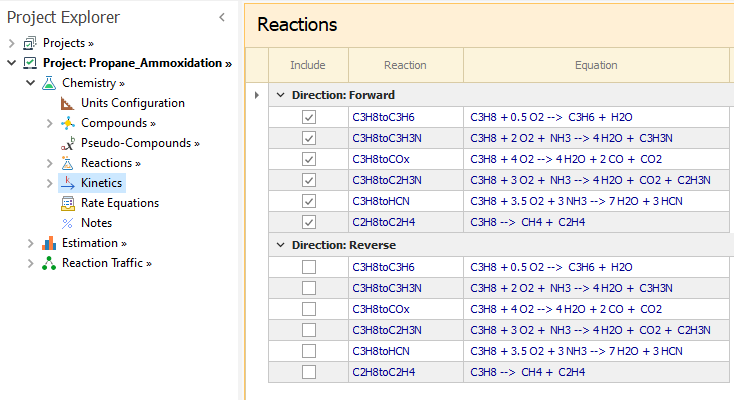
Forward direction only is enabled for all reaction in Chemistry → Kinetics node:
|
|---|
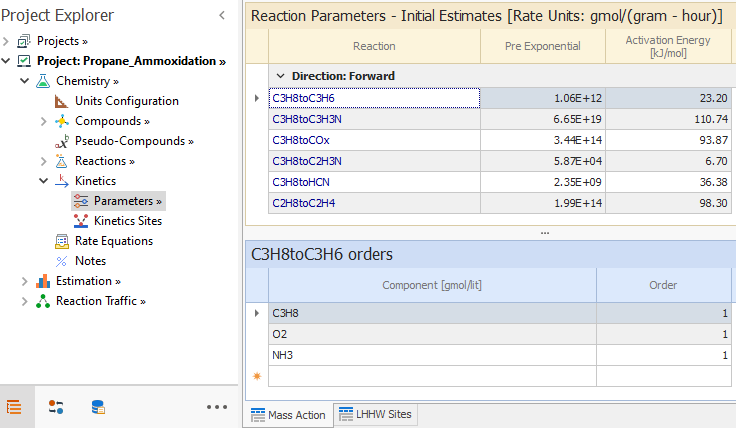
Then we define the kinetics parameters values for the reaction.
We first load the PreExponential and Activation Energy values for the parameter k that belongs to the numerator of the previous equation.
That is done in the Kinetics → Parameters node, as shown in the image below:
|
|---|
Notice that the propane, oxygen and ammonia orders are 1.
The following step is to define the sites, that is done in the LHHW Sites tab of Chemistry → Kinetics → Parameters node.
In this example, two sites are needed:
The first Site is: 1 + KC3H8CC3H8 , where:
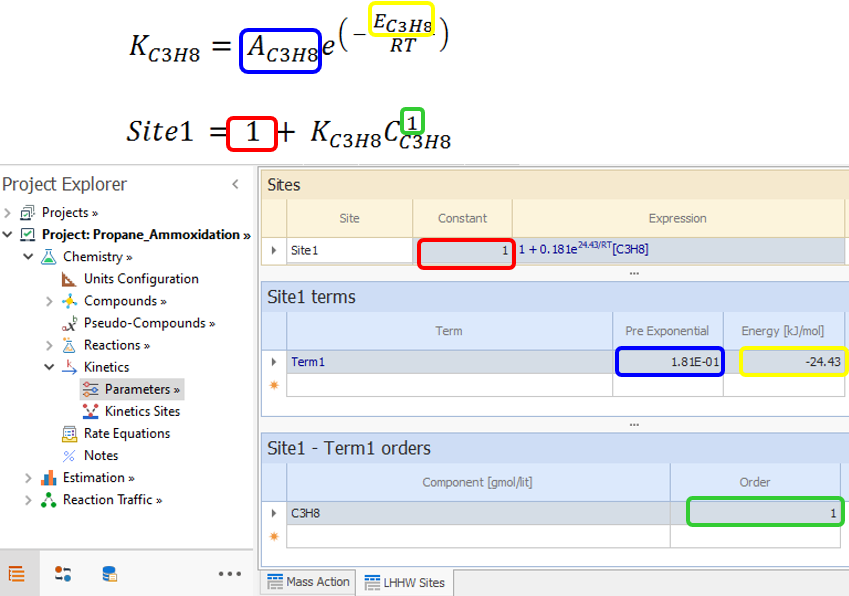
|
|---|
The second site has two terms: Site2 = 1 + KO2CO2 + KNH3CNH3
Enter the first term :
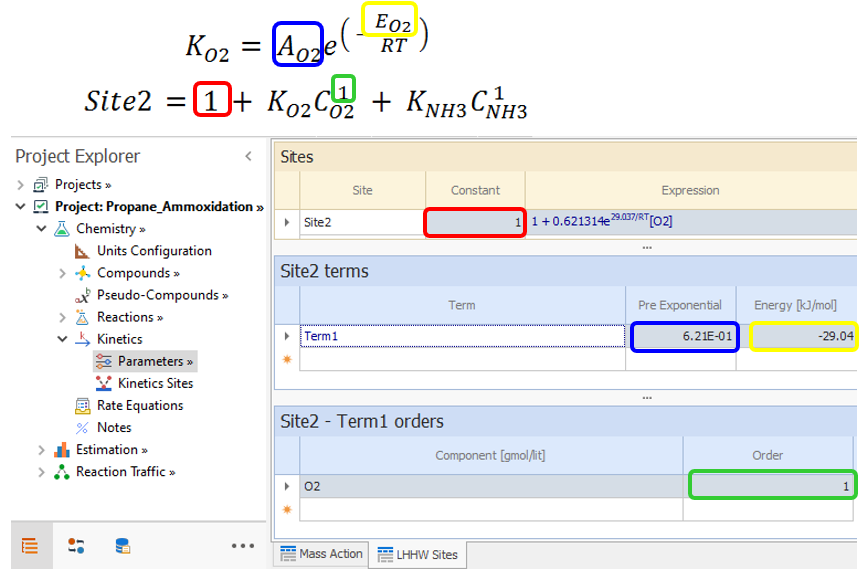
|
|---|
Enter the second term:
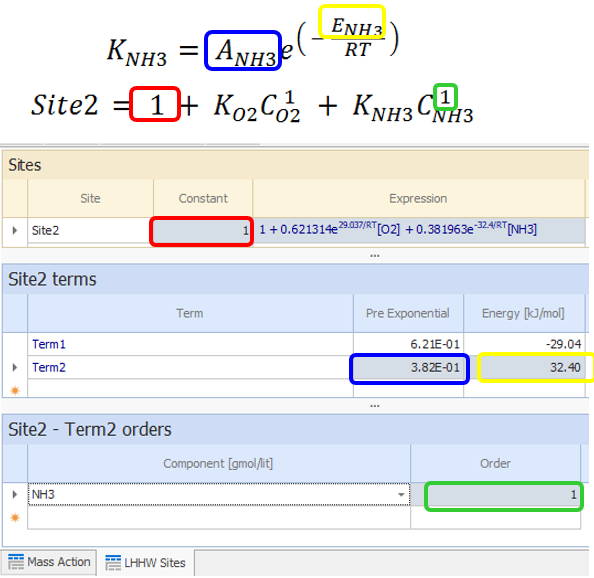
|
|---|
After the Site definition is done, they must be associated with the appropriate reaction in Chemistry → Kinetics → Kinetics Sites node.
First, the sites are selected for reaction C3H8toC3H6 in the Kinetics Site tab:
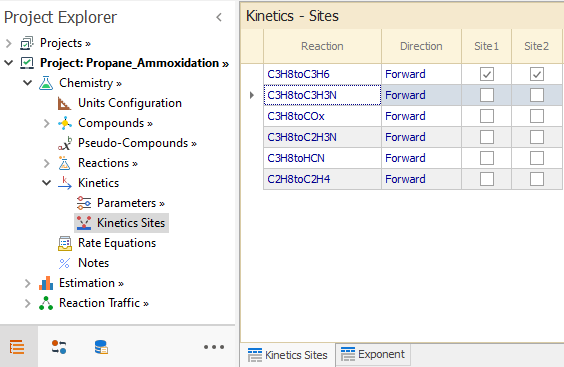
|
|---|
Finally, the exponent for each Site in the C3H8toC3H6 reaction is entered in the Exponents Tab:
|
|---|
This completes the kinetics setup for the first reaction.
Top of Topic
Go back to: